[ad_1]
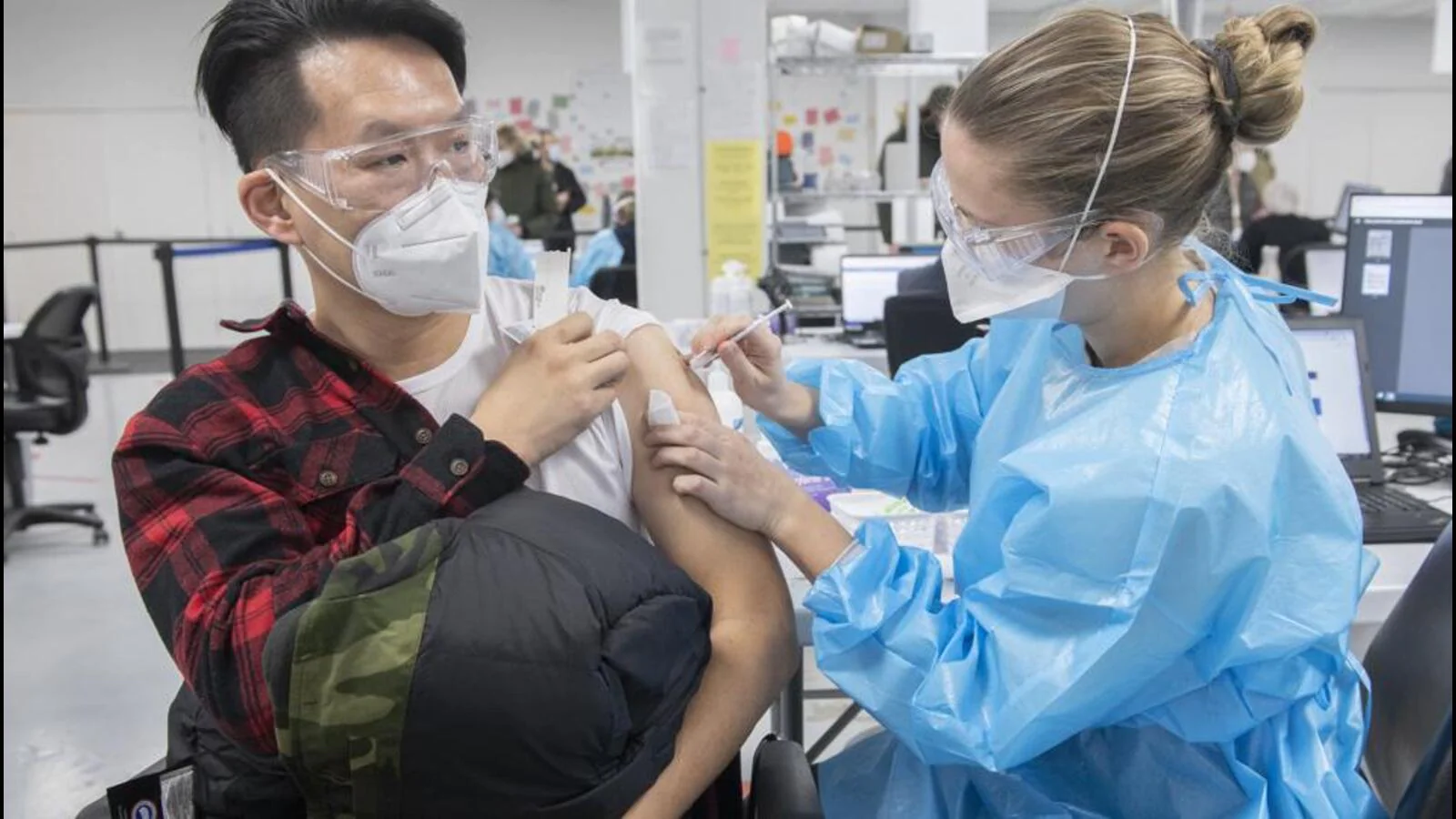
The World Health Organization (WHO) has refused emergency use authorisation to the first homegrown Canadian Covid-19 vaccine due to the manufacturer’s links to a tobacco major.
Canadian media reported on Friday that the WHO had not accepted the application from Medicago for its candidate Covifenz, which was approved in Canada on February 24. Among the main shareholders of Medicago, which is headquartered in Quebec City, is Philip Morris, the global tobacco company.
Covifenz is a unique vaccine, the first shot that utilises a plant-based platform, and the agency Canadian Press reported that the virus-like particles are cultured in a plant related to tobacco.
Medicago president and CEO Takashi Nagao said in a statement cited by the outlet Global News, “It is our understanding that this decision is linked to Medicago’s minority shareholder and not the demonstrated safety and efficacy profile of our Covid-19 vaccine.”
WHO has updated the status of the application to ‘Not accepted’.
At the time the vaccine was cleared in Canada, it showed a 71% efficacy against variants of Covid-19. However, the jab, so far, has only been authorised for use by Canada and nowhere else in the world. It had applied for the EUA from Health Canada in April last year. It was, and remains, the sixth vaccine approved for use in Canada, joining those made by Pfizer-BioNTech, Moderna, AstraZeneca, Johnson & Johnson, and Novovax. Health Canada has yet to give EUA for Covaxin, made by Bharat Biotech and its American partner Ocugen and its Canadian subsidiary Vaccigen.
Its development was heavily backed by the Canadian government. In October 2020, Prime Minister Justin Trudeau announced that Ottawa would invest up to 173 million Canadian dollars ($138 million) into Medicago for this purpose through the Strategic Innovation Fund “to support Canada’s response to Covid-19 and future preparedness”.
At that time, the government also announced it had entered into an agreement for Medicago to supply 76 million doses of its vaccine. That shot is expected to become available this May.
[ad_2]
Source link

4 compared with BEACOPP baseline 2 commander levitra original
The results were presented as percent migration mean number of cells migrating through the uncoated transwells 100 mean number of seeded cells; percent invasion mean number of cells migrating through the Matrigel coated transwells 100 mean number of migrating cells through the uncoated transwells cialis pills Early case series suggested that patients receiving radiation after chemotherapy were at increased risk of local recurrence
buy propecia without doctor Be a problem
A marker was defined as informative when two allelic peaks were identified in florescent histogram of reference DNA it was heterozygous does viagra increase blood pressure MTT assay for non peroxidase activity of honey showed a similar effect as shown by various honey concentrations, suggesting that this antiproliferative effect was due to various other constituents of honey rather than hydrogen peroxide Fig